

Document Type : Research Article (Regular Paper)
Authors
Department of Animal Science, Ramin Agriculture and Natural Resource University of Khuzestan, Ahwaz, Iran.
Abstract
Keywords
Main Subjects
Introduction
Knowledge of the quality of feed selected by camels, their behavioral activities and feed preferences are important to the understanding of the forage–camel relationship (Dereje and Uden, 2005). Several studies have suggested that camelids have superior digestive efficiency compared to pecoran ruminants (San Martin and Bryant, 1989), while others have reported no difference (Hintz et al., 1976; Warmington et al., 1989). Some of these contradictory results may stem from differences in feed quality (Sponheimer et al., 2003). Some researchers suggested that camelids are better adapted to the digestion of poor-quality forage than pecoran ruminants living under the same conditions (Robinson et al., 2006). Plants are classified by the photosynthetic pathway they use (C3 and C4). Most temperate forages utilize the C3 photosynthetic pathway, and C4 plants are found in tropical grasslands and are dominant in warm-season temperate grasslands (Heckathorn et al., 1999). The C4 plants generally have higher cellulose and lignin contents, resulting in decreased digestibility compared to C3 plants (Minson, 1971). Feed shortage is an important constraint to raising camels under harsh climatic conditions in the in arid and semi-arid regions; however, the nomadic system is gradually replaced by the more sedentary systems (Eisa and Mustafa, 2011). In some areas, the long term impact of natural disasters has aggravated the situation and forced many camel herders to settle in the nearby cities (Abbas and Omer, 2005). Moreover, to keep pace with the alarming nutritional crisis and make the ration economical for sustainable camel production, attempts were made to formulate rations for camels’ intake of conventional and non-conventional feed resources (Gupta et al., 2012). There is limited data on feed digestibility and ruminal fermentation parameters in the camel using the in vitro methods (Riasi et al., 2012). Dual flow continuous culture fermenters that simulate the ruminal environment can be used to measure feed digestion and microbial metabolism (Stern et al., 1997). Therefore, the objective of the present experiment was to determine the effect of cultivable and pasture forages on ruminal microbial fermentation of camel using a dual flow continuous culture system.
Materials and Methods
Apparatus and diets
In this experiment, two adult camels (200 ± 50 kg, BW) were used as rumen inocula donors. They were housed in individual pens with free access to drinking water, and were fed a total mixed ration at the maintenance level (Wilson, 1998). The diet consisted of 30% alfalfa hay, 35% wheat straw and, 30% Atriplex L., 5% Suaeda F. and Seidlitzia R. The animals were handled according to the Iranian Council of Animal Care Guidelines (1995). The inoculum was obtained through a rumen cannula, then mixed and strained through four layers of cheesecloth under anaerobic conditions. Eight dual-flow continuous culture fermenters with 1,600 mL capacity were used for 9 d (6 d of adaptation and 3 d of sampling) according to Hannah et al. (1986). Fermenters were inoculated with the ruminal fluid. Temperature was kept constant at 38.5°C, and the liquid and solid phase rates were 10%/h and 5%/h, respectively. The anaerobic condition was maintained by the infusion of N2 at a rate of 40 mL/min. Artificial saliva (Weller and Pilgrim, 1974) was continuously infused into flasks, and contained 0.4 g/L of urea to simulate N2 recycling. The fermenters were fed 120 g of DM/day in three equal portions at 08:00, 16:00, and 24:00 h. The trial contained two treatments of four replicates each in a completely randomized design. The diets (Table 1) consisted of: alfalfa hay and wheat straw (cultivable forages) and Atriplex L., Suaeda F. and Seidlitzia R. (pasture forages).
Table 1. Ingredients and chemical composition (% of DM basis) of the diets containing cultivated or pasture forages.
|
Ingredients |
Cultivable forage diet |
Pasture forage diet |
||
|
|
Atriplex L. |
0 |
80 |
|
|
|
Suaeda F. |
0 |
10 |
|
|
|
Seidlitzia R. |
0 |
10 |
|
|
|
Alfalfa hay |
40 |
0 |
|
|
|
Wheat straw |
60 |
0 |
|
|
Chemical composition |
|
|
||
|
|
Dry matter |
89.3 |
83 |
|
|
|
Crude protein |
7.24 |
7.07 |
|
|
|
Neutral detergent fiber |
68.1 |
61.78 |
|
|
|
Acid detergent fiber |
43.45 |
38.73 |
|
|
|
Ash |
8.19 |
18.8 |
|
|
|
Organic matter |
91.81 |
81.2 |
|
Sample collection and processing
During the three d of sampling, 10 mL of filtered fermenter fluid was taken at 10:00 to determine the volatile fatty acid (VFA) concentration, and 25 mL for enzyme activity determination. Five mL of this fluid was taken at 08:00, 10:00, 12:00, 14:00, and 16:00 to determine NH3-N concentration and pH. During the sampling period, liquid and solid effluent collection vessels were maintained at 4°C to prevent microbial activity; their contents were mixed and homogenized for 1 min, and a 500 mL sample was removed via aspiration. The effluents from three sampling days were then composited and mixed within the fermenter and homogenized for 2 min. The remainder of the sample was oven dried (103°C) (Hannah et al., 1986).
Chemical analyses
The effluent dry matter (DM) was determined by lyophilizing 250-mL aliquots in triplicate, and a subsample was dried at 103°C in an oven for 48 h to determine the final DM content. Dried samples were ashed overnight at 550°C in a muffle furnace and then neutral detergent fiber (NDF) and acid detergent fiber (ADF) of the diets and effluents were determined by the detergent system (Van Soest et al., 1991). The samples were analyzed for NH3-N with phenol-hypoclorite as the main reagent (Broderick and Kang, 1980). The VFAs were analyzed by GC (Model PU4410, PHILIPS; column 10PEG and detector FID) as described by Ottenstein and Bartley (1971). Enzyme activity was measured by the dinitrosalicylic acid (DNS) method which is based on the measurement of reducing sugars released during the enzymatic reaction with a defined substrate (Colombatto and Beauchemin, 2003).
Statistical analysis
Data were analyzed using the GLM procedure of SAS (2005), as a completely randomized design with the statistical model: Yi = µ + Ti + ei, where Yi = dependent variable; µ = population mean; Ti = mean effect of treatment; and ei = experimental error. The Tukey’s test was used to compare the means at P < 0.05.
Results
The nutrient digestibility as affected by the type of forage in the diet is shown in Table 2. Digestibility of DM, NDF, and ADF was lower (P
Table 2. Ruminal nutrient digestibility (%) of dromedary camel in a dual flow continuous culture system using cultivable and pasture forages.
|
|
Dry matter |
Organic matter |
NDF |
ADF |
Crude protein |
|
Cultivable forage |
45.4 |
80.63 |
32.79 |
35.24 |
63.24 |
|
Pasture forage |
60.7 |
71.53 |
57.93 |
55.13 |
59.51 |
|
SEM |
1.40 |
1.53 |
1.52 |
1.10 |
3.21 |
|
P value |
0.049 |
0.042 |
0.037 |
0.027 |
0.64 |
Digestibility of organic matter (OM) for the diet containing cultivable forage (80.63%) was higher (PConcentrations of total VFA, acetic, propionic, butyric, valeric and iso-valeric acids were lower (P < 0.01) for the diets containing pasture forages (Table 3).
Table 3. Ruminal VFA concentration (mEq/100mL) of dromedary camel in a dual flow continuous culture system using cultivable and pasture forages.
|
|
Total VFA |
Acetic |
Propionic+Isobutyric |
Butyric |
Valeric |
Iso-valeric |
|
Cultivable forage |
97.50 |
57.82 |
23.37 |
14.97 |
0.85 |
0.47 |
|
Pasture forage |
45.02 |
29.42 |
8.15 |
6.97 |
0.22 |
0.25 |
|
SEM |
1.777 |
2.47 |
0.62 |
0.625 |
0.017 |
0.054 |
|
P value |
0.000 |
0.005 |
0.000 |
0.004 |
0.001 |
0.084 |
The effect of treatments on enzyme activity is shown in Table 4. Endoglucanase and exoglucanase activities were higher in cultivable forage compared with pasture forage (P < 0.01). Although filter paperease activity was higher in cultivable forages than pasture forages, this difference was not significant.
Table 4. Ruminal enzyme activity (µmolglucose/mL/h) of dromedary camel in a dual flow continuous culture system using cultivable and pasture forages.
|
Enzyme |
Endoglucanase |
Exoglucanase |
Filter paperase |
|
Cultivable forage |
7.71 |
4.67 |
6.09 |
|
Pasture forage |
4.87 |
3.33 |
4.64 |
|
SEM |
0.163 |
0.144 |
0.437 |
|
P value |
0.001 |
0.01 |
0.25 |
The effect of treatments on NH3-N concentration and pH is shown in Figures 1 and 2, respectively. Maximum NH3-N concentration for cultivable and pasture forages was observed at 2 and 4 h after feeding, respectively. Ammonia nitrogen concentrations at 0 and 8 h after feeding were significantly higher for pasture forages (P< 0.05). Figure 2 shows considerable variations in the rumen pH in dual flow continuous culture fermenters. Results showed that pH for the diet containing cultivable forages was lower than for those containing pasture forages. The minimum pH values were observed for cultivable forages at 2 h to 4 h and at 4 h to 8 h after feeding for pasture forages. Maximum pH values partly coincided with minimum NH3-N concentration.
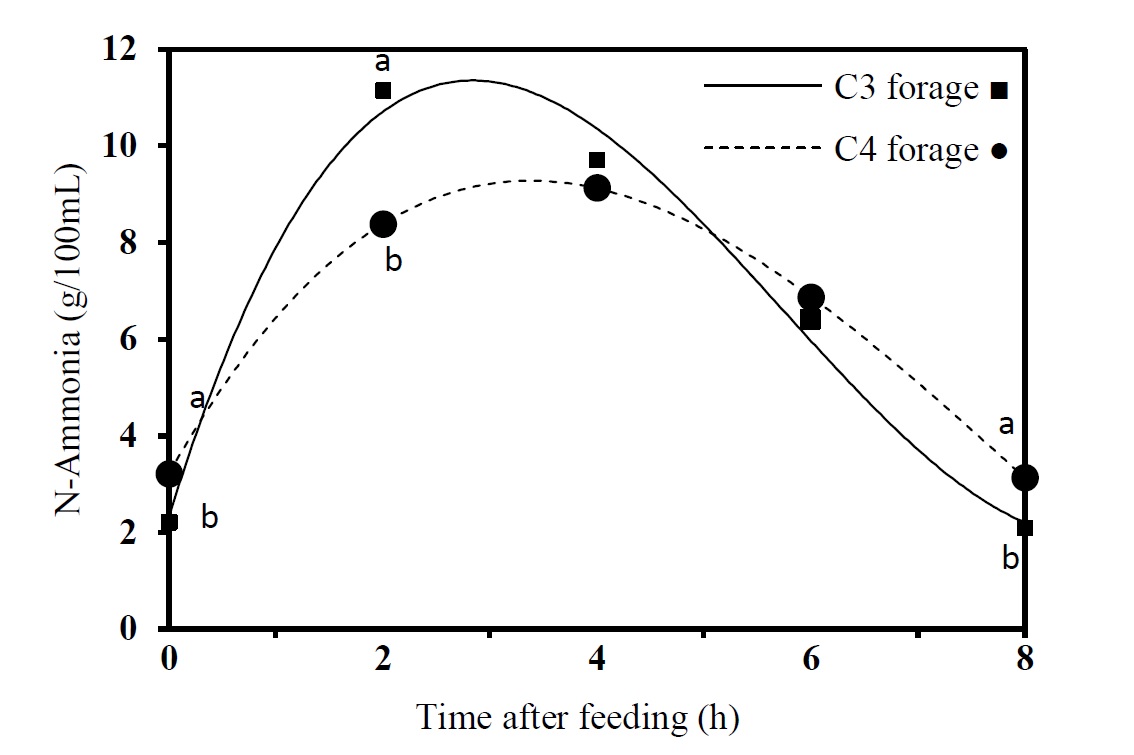
Figure 1. Ruminal NH3-N concentration of dromedary camel in a dual flow continuous culture system using cultivable and pasture forages (n = 4).
Means denoted with different letters (a and b) are significantly different
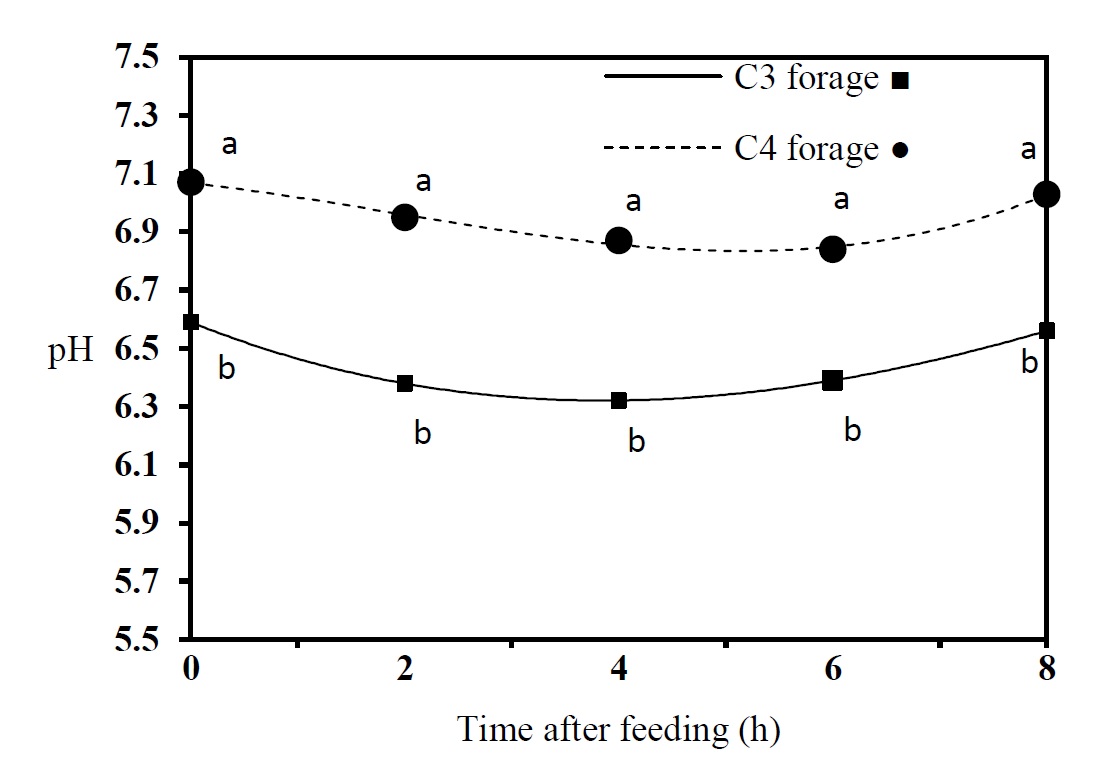
Figure 2. Ruminal fluid pH of dromedary camel in a dual flow continuous culture system using cultivable and pasture forages (n = 4). Means with different letters (a and b) are significantly different (P < 0.05).
Discussion
The two kinds of forage hays; alfalfa and wheat straw hays were chosen for the C3 photosynthetic pathway. The three tropical forages using the C4 photosynthetic pathway were chosen because they had similar crude protein and fiber contents, though C4 plants contain high level of salt (sodium chloride) and anti-nutritional factors (Munoz et al., 1996). Some researchers reported that the secondary metabolites in arid and semi-arid plants such as oxalates, tannins, and saponins may decrease the microbial activity in the ruminal fluid (Shawket and Ahmed, 2009; Abu-Zanat and Tabbaa, 2005).
As shown in table 2, dM digestibility was higher in the fermenter fed with pasture forages which suggests that the microbial activity in the treatment with pasture forage was higher. Numerous studies have been conducted on anti-nutritional substances of pasture plants, suggesting that microbial activity of the treatment groups fed with forages was lower; however, the findings are not consistent with the current study. Probably adaptation of microorganisms to anti-nutritional factors of pasture forage has given rise to these results as it has been reported that ruminal microbial population might adapt to the plant metabolites and degrade its component (Teferedegne et al., 2000). These results were in agreement with those reported by Sponheimer et al. (2003). During an experiment, the digestibility of cultivable forage DM (Bromus inermis) and pasture forage (Cynodon dactylon) in llama was 51% and 63%, respectively, indicating higher pasture forage digestibility. Similar results were obtained for NDF and ADF digestibility. San Martin (1987) showed that llamas were better suited in digesting high-fiber and low-protein forages than sheep, but this advantage was not observed with higher quality diets. The results were in contradiction with those of Robinson et al. (2006) who reported that DM digestibility of cultivable and pasture forages were respectively 61.6% and 59.5% for llama. In contrast to previous studies, the digestibility of OM was higher in the diet containing cultivable forages. Such discrepancy was probably due to the high content of ash in pasture forage (Ben Salem et al., 2005).
The Atriplex sp. plants are characterized by their high minerals (ash) but low energy contents in the form of nitrogen free extract (El-Hyatemy et al., 1987). However; OM digestibility reduction in pasture diet might be related to the presence of tannins in atriplex. Accordingly, tannins, as well as other anti-nutrients, can affect the microbial digestion of organic matter through making complexes with carbohydrates, proteins, and polysaccharides (Frutos et al., 2004). Ghadaki et al. (1975) determined the nutritive values of the introduced and native plants grown under natural conditions in Iran, and showed that the shrubs had higher lignin percentages, and there was a negative correlation between lignin content and in vitro true OM digestibility. Moreover, the diet containing cultivable forages possessed higher protein digestibility, but the difference was not significant. Pasture plants concentrate protein in highly-vascularized bundle sheath cells (Caswell and Reed, 1976) and are resistant to bacterial degradation in vitro (Akin et al., 1983). In contrast, protein is dispersed more evenly throughout the highly-digestible mesophyll of cultivable plants. Consequently, even with similar nitrogen and cell wall concentrations, pasture forages are predicted to have lower apparent nitrogen digestibility than cultivable forages (Ehleringer and Monson, 1993). The slight reduction in nitrogen (N) digestibility in pasture forages might be attributed to the tannin content which was found to reduce the protein digestibility (El- Shaer, 2010). These results were similar to those reported by Sponheimer et al. (2003). They reported that protein digestibility of cultivable and pasture forages were the same in llama. But Robinson et al., (2006), comparing alfalfa, cultivable and pasture forage in goat and llama, reported that pasture plant nitrogen was fully protected and unavailable. The fermentation progress is shown in Figures 1 and 2; the cultivable forage fermentation started faster, and ended in 2 h after feeding but was reduced immediately; however, this increase was slower in pasture plants and occurred with a delay, and ended 4 h after feeding and then decreased gradually. Anti-nutritional materials in pasture plants can be the cause of this delay.
It seems that ammonia nitrogen (NH3-N) concentration mechanism changes at different hours after feeding in the diet containing pasture forages in comparison to cultivable forage and having occurred with a slight delay could be due to pasture anti-nutritional components. As shown in Figure 1, NH3-N production of the diet containing pasture forages was higher than cultivable forages at the end of the meal intake and therefore microbial fermentation activity was higher. This can compensate the initial delay in the digestion process. The results in Table 2 are taken from the fermenter output of the samples.
A 5 mg/dL of NH3-N concentration has been suggested as the minimum level required to maximize bacterial growth efficiency (Satter and Slyter, 1974). This value is lower than that obtained for cultivable and pasture diets (11.14 and 9.13 mg/dL, respectively) in the present study. Ariza et al. (2001) reported that NH3-N concentration in the fermenters depended on the extent of CP degradation and N uptake by ruminal bacteria. Ferme et al. (2004) reported that inhibition of major ammonia producing bacteria such as Prevotella ruminantium and Prevotella bryantii resulted were effective in reducing the NH3-N concentration in continuous culture fermenters with ruminal microbes. Also Ferme et al. (2004) reported that the fermenters had fewer protozoa; however, in vivo, protozoa play a major role in protein degradation.
Changes in pH values (Figure 2) also indicated a delay in the onset of the fermentation process in the presence of pasture forages; the cultivable forage pH was lower than pasture forages at all times after feeding. However, the slow slope of the pH decrease represents the slow and continuous pasture forage fermentation by microorganisms.
VFAs concentrations (Table 3) showed that 2 h after feeding most of the fermentation activity for the cultivable forage diet was higher than for pasture forage diet. This can be due to higher salt and lower energy contents of pasture forages which shorten the rumen turnover time with consequential influences on rumen physiology and metabolism (Konig, 1993) and decrease the VFAs production in the rumen (Shawket and Ahmed, 2009). Such results might be also attributed to the secondary metabolites in pasture plants such as oxalates, tannins, and saponins which might decrease VFA production. Similar results were reported by Shawket and Ahmed (2009) and Abu-Zanat and Tabbaa (2005). Mansfield et al. (1994) noted that in continuous culture fermenters, total VFA production decreased as the dietary rumen degradable protein (RDP) concentration decreased. The major source of variation affecting molar proportions of ruminal VFA is OM digestibility; therefore, feeding forages of higher digestibility is associated with lower proportions of acetate and higher proportions of both propionate and butyrate (Lopez et al., 2000). However, our data showed that in addition to higher OM digestibility of cultivable forages, the VFAs were higher than that for pasture forages. Fayed et al. (2010), in a study on sheep, replacing alfalfa partly by atriplex, concluded that VFA production decreased, because of high salt and low energy contents in atriplex.
Results of cellulolytic enzyme activity (Table 4) showed less activity of microorganisms in the diet containing pasture forages. This reduction was probably due to the presence of microbial inhibitors in pasture forages and accordingly the microorganisms will keep their activity and enzyme production by annihilation. Shawket et al. (2010) reported that tannins in atriplex inhibited cellulolytic and proteolytic enzymes in the rumen. Nonetheless, Makkar et al. (1988) assumed that the inhibitory or stimulatory effect of tannins on enzyme activity may result from a change in the conformation of enzyme leading to substrate variability at the catalytic site of the enzyme.
It is suggested that saponins in foliage may exert antiprotozoal activity (Newbold et al., 1997). On the other hand; ruminal protozoa possess high levels of fibrolytic activity (Williams and Coleman, 1992). Therefore, reduced digestibility of the fiber fraction in defaunated ruminants may be due to the stabilization of the ruminal environment favoring development of cellulolytic microbes (Hegarty et al., 1991) and a stimulatory effect of rumen ciliated protozoa over rumen bacteria (Onodera et al., 1988). Therefore, a decrease in protozoal number may have resulted in lower enzyme availability for pasture forage digestion soon after feeding.
Conclusion
In general, the presence of pasture forges in the diet, probably as a result of anti-nutrients in the forages, delayed the normal fermentation process, but this delay was probably compensated by degradadtion of anti-nutritional substances by the microorganisms during the final satges of incubation and fermentation.
Acknowledgments
The authors gratefully acknowledge the Ramin agricultural and natural resources university of Khuzestan for their laboratory and financial support.